Can a Non Polar Molecule Contain Polar Bonds
A molecule that has only nonpolar bonds and no polar bonds cannot be polar. Meaning the molecule is non-polar because were missing one side having a positive charge.

Aim What Are Polar Bonds And Polar Molecules Polar And Nonpolar Bonds There Are Two Types Of Covalent Bonds Nonpolar Covalent Bonds Equal Share Of Ppt Download
How do you figure out if a molecule is polar or nonpolar.
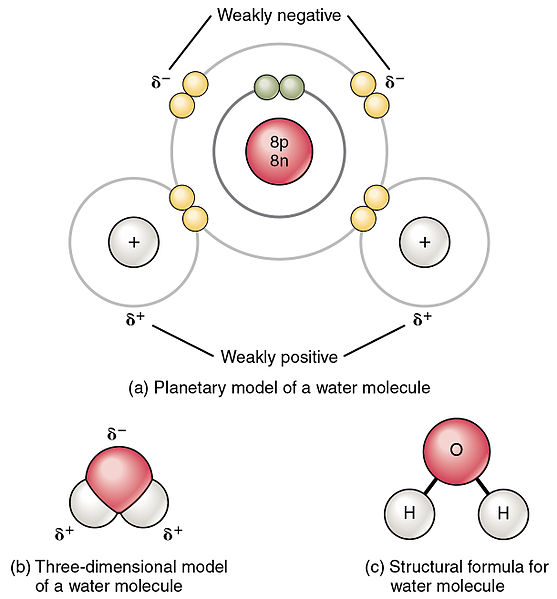
. A polar molecule always contains polar bonds but some molecules with polar bonds are nonpolar. HCL is a polar molecule as chlorine has a higher electronegativity than the hydrogen. If the polar bonds are evenly or symmetrically distributed the bond dipoles cancel and do not create a molecular dipole.
Are all molecules polar. This effect can be seen in a number of linear trigonal planar and tetrahedral substances. A molecule may be nonpolar either when.
The non-polar covalent bond is formed between the two atoms by sharing of electrons between the two atoms. Hence it can be concluded that the molecule is a non polar molecule having polar bonds. The bonds in the molecule are polar because electronegativity causes one side of the bond to be positive and the other side to be negative.
A molecule can possess polar bonds and still be nonpolar. A molecule can possess polar bonds and still be nonpolar. The polarity of a molecule depends on the net dipole moment of the molecule.
Explain your answer with an example. Expert Answer 100 1 rating A polar bond is a bond where the constituent atoms have different electronegativity making unequal distribution of electronexample HCl 1A molecule can be non polar based on two reason one all the constituent molecule will be of same electrone. A molecule that consists only of nonpolar bonds and no polar bonds cannot be classified as polar in nature.
Diatomic ionic and polar covalent molecules are polar molecules. Lets say you have a linear-shaped molecule. The polarity of a molecule depends on the net dipole moment of the molecule.
In non-polar covalent bond the electronegativity difference is not-observed. If the molecule is linear in. Can a nonpolar bond be polar molecule.
The presence of polar bonds does not automatically mean that. O C O C Cl Cl Cl Cl B F F. YES a nonpolar molecule can contain polar bonds.
Likewise people ask what is an example of a nonpolar molecule with polar bonds. It is possible that a non polar molecule contain polar bonds. The polar covalent bond between the atoms forms a polar covalent compound.
Thus bond become polar because one end is partial positive and other is partial negative. Can a nonpolar molecule have polar bonds. How can a molecule be nonpolar overall and still contain polar bonds.
View this answer YES a nonpolar molecule can contain polar bonds. Expert Answer 100 4 ratings yes it is p. What bonds can form between nonpolar molecules.
Polar Molecule A molecule in which the bond dipoles present do not cancel each other out and thus results in a molecular dipolesee below. Yes a non polar molecule can have a polar bond as long as it has another opposite polar bond to counter the first. Click to see full answer.
A polar bond is one in which electron density is unevenly distributed between the bonding atoms due to significant difference in electronegativity between the covalently bonded atoms. How can a non-polar molecule contain polar bonds. Hard Answer Its all because of electronegativity.
A molecule that CONTAINS nonpolar bonds on the other hand is distinct since it can also include polar bonds. What is non polar molecule. If the polar bonds are evenly or symmetrically distributed the bond dipoles cancel and do not create a molecular dipole.
The overall atom is non-polar because there are two negatively charged sides instead of one positive side and one negative side. If we look at just the bond between the carbon and the oxygen then we see a polar bond. Is HCL polar or nonpolar.
See the answer Can a non-polar molecule contain polar bonds. View the full answer Transcribed image text. The larger the difference in electronegativity between the two atoms the more polar the bond.
Now you can see that there are no electrons around the central atom. If the arrangement is symmetrical and the arrows are of equal length the molecule is nonpolar. If the polar bonds are evenly or symmetrically distributed the bond dipoles cancel and do not create a molecular dipole.
A on polar molecule is when there is an equal. Advertisement New questions in Science the way that something fells when you touch it from the given group of picturesidentify which one does not belong to the groupand explain why. So it is non polar.
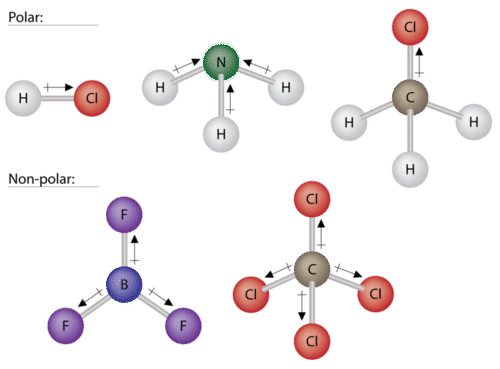
But molecules containing more than two atoms can be polar too. So the correct answer is hydrogen peroxide. For example the three bonds in a molecule of BF3are significantly polar but they are symmetrically arranged around the central boron atom.
In a few cases a molecule may have polar bonds but in a symmetrical arrangement which then gives rise to a non-polar molecule such as carbon dioxide. Thus a non polar carbon dioxide molecule have polar bond. Both the atoms have the same electronegativity value.
A molecule that comprises nonpolar bonds can nevertheless be classified as polar if it also. On taking the resultant of these two vectors it is found that the NET DIPOLE MOMENT is zero. A molecule that contains nonpolar bonds can be polar as long as it also contains polar bonds.
A non-polar molecule may contain polar bonds because polarity also has to do with the geometry of the molecule. In many cases however the presence of polar bonds dipoles does not result in a permanent dipole on the molecule as there are other polar bonds dipoles in the same molecule which have the effect of cancelling each other out. Cancellation depends on the shape of the molecule or Stereochemistry and the orientation of the polar bonds.
However a molecule that CONTAINS nonpolar bonds is different because it can contain polar bonds. Yes a molecule can be nonpolar when it contains polar covalent bonds because think about it. A molecule can possess polar bonds and still be nonpolar.
This is because oxygen is slightly more electronegative than carbon. In this case lets use C O2.
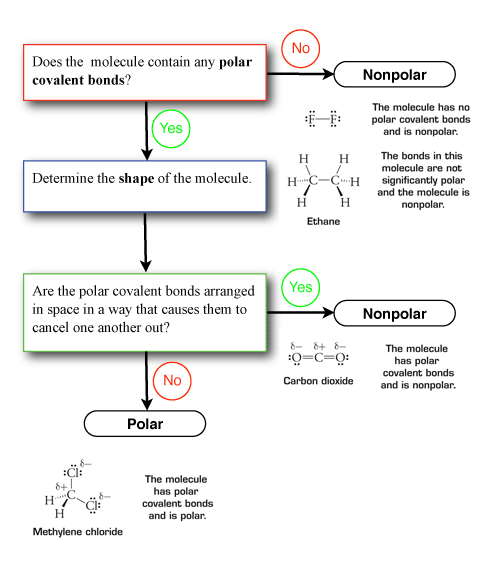
Unit 1 Elaboration Molecular Polarity

Is It Possible For A Molecule To Have A Polar Bond But Have An Overall Polarity Of Nonpolar Quora
5 3 Polarity And Intermolecular Forces Chemistry Libretexts
Difference Between Polar And Nonpolar Molecules Definition Formation Properties Examples
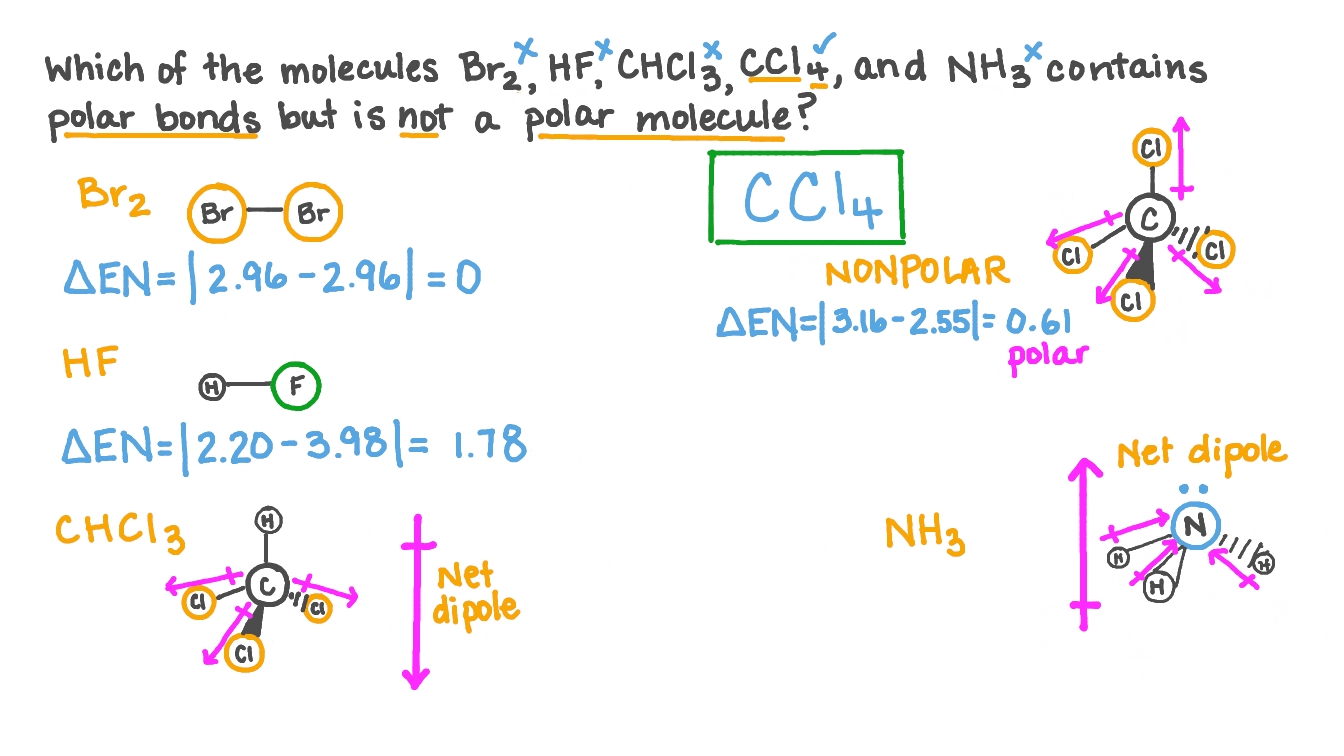
Question Video Determining The Molecule That Contains Polar Bonds But Is Not A Polar Molecule Nagwa
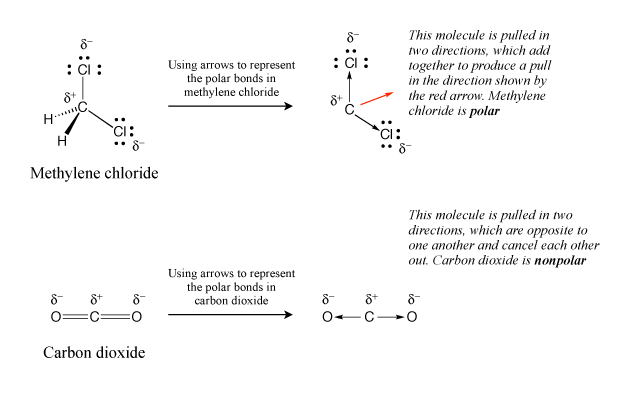
Unit 1 Elaboration Molecular Polarity

Polar And Non Polar Molecules Vce Chemistry

Polar Vs Non Polar Bonds Molecules Chemtalk
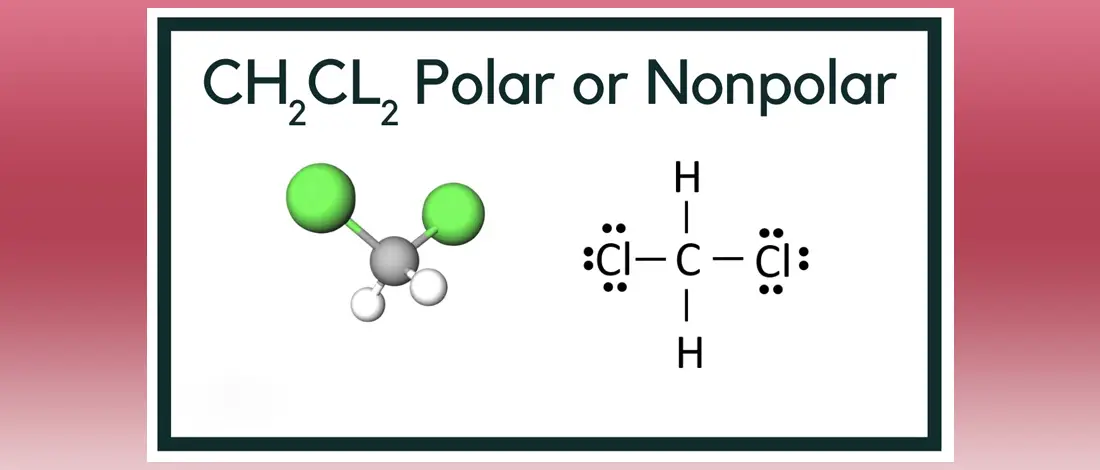
Is Ch2cl2 Polar Or Nonpolar All You Need To Know

5 7 Polarity Of Molecules Chemistry Libretexts

Polar Molecules Section Ppt Download

Nonpolar Covalent Bond Definition And Examples



Comments
Post a Comment